Nail polish contains several ingredients that give it its strong odor.
Some of those ingredients are the solvents used to keep the plastic liquid until it dries on your toenails. Butyl acetate, ethyl acetate, and toluene are examples. Butyl acetate gives bananas their smell, and is used to flavor candies.
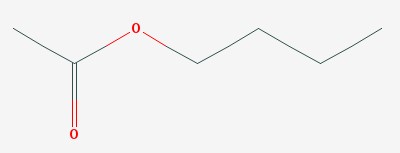
Butyl acetate
Ethyl acetate is another similar ester that smells like pears. When these two molecules are present in huge amounts, they can have a very strong odor.
Toluene is derived from petroleum, and give paint thinner its characteristic smell. It is also used as the fluid that fuels cigarette lighters.
Nail polish may also include camphor, a strong smelling molecule that is used to keep the plastic flexible. Camphor is now made synthetically, but it is found in the camphor laurel tree that grows in China and Borneo. It is sometimes used as a moth repellant (the tree probably produced it as an insecticide). It give Vicks VapoRub its strong scent.
Nail polish remover also has an interesting smell. It is mostly acetone, a strong organic solvent that is good at dissolving many plastics, glues, and paints.
