There are two molecules that go by the common name ammonia. This first is a gas, made from a nitrogen atom and three hydrogens.
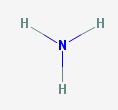
Ammonia
Ammonia gas is also known as anhydrous ammonia, which means ammonia without water. Ammonia reacts with water in the environment very easily, so almost all of the ammonia you will ever encounter is actually ammonium hydroxide.
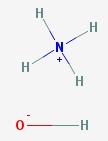
Ammonium hydroxide
The ammonia molecule steals a hydrogen nucleus (a proton) from water. This makes the ammonium ion NH4+ and leaves the hydroxyl ion OH- as the only thing left of the water molecule. Since they have opposite charges, they attract one another and hang around the same neighborhood. Household ammonia, used as a cleaning agent, is actually water and ammonium hydroxide.
Ammonia is a base, like lye (sodium hydroxide). Like lye, it can react with oils and fats to form soaps. As a cleaner, ammonia turns fats and oils on glass or tile surfaces into soap, and the water in the ammonia solution dissolves the soaps so the sponge or paper towel can carry them away. What is left is a solution of ammonium hydroxide, which then completely evaporates, leaving no streaks on the surface.
Animals make ammonia from proteins in the food they eat, and they use the ammonia to neutralize acids in their urine. This is why a crowded barn or stable has a strong ammonia scent.
