Zanfel™ is a cream that contains several strong surfactants (detergents) and tiny polyethylene beads.
The main detergent in Zanfel™ is sodium lauroyl sarcosinate.
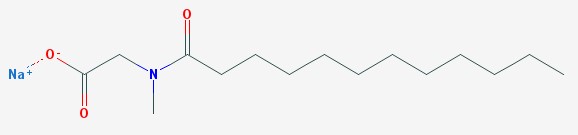
Sodium lauroyl sarcosinate
This detergent has a capability we have not discussed in other detergents. It is a penetration enhancer. That means it helps other molecules to penetrate deeper into the skin. It is an ionic surfactant (you can see the plus sign by the sodium ion, and the minus sign by the oxygen). It is derived from coconut oil, and cleans without completely stripping the skin of all oils.
The second detergent in Zanfel™ is nonoxynol-9.

Nonoxynol-9
Nonoxynol-9 is a non-ionic detergent. We saw these earlier when we discusses laureth-12 in Shout™ stain remover. The long chain of nine ethylene oxide groups is water loving, and the long fatty acid chain at the bottom is oil loving.
When we discussed another surfactant, benzalkonium chloride, the antiseptic in Bactine™, we mentioned that some surfactants are good at breaking up cell walls and killing microbes. The same thing happens with nonoxynol-9. It is used in contraceptives to kill sperm. In Zanfel™ it is used to break up the poison urushiol that causes the poison ivy rash.
Nonoxynol-9 is a polyethylene glycol. The long chain with all ethylene oxide units (two carbons and an oxygen) is the polyethylene part (poly means many). Another polyethylene glycol is C12-15 Pareth-9, and it is the third detergent in Zanfel™.
To make these detergents work better in hard water, sodium EDTA is added. It grabs onto magnesium and calcium ions in the water, and keeps them from interfering with the detergents.
To keep the detergents from spoiling, a bactericide called quaternium-15 is used. It releases formaldehyde to kill germs.
Another surfactant, triethanolamine (which is three ethylene molecules all attached to a central nitrogen atom) helps make the urushiol soluble in water. It also adjusts the acidity of the product, and neutralizes fatty acids.
