There are two hydrogen sulfates.
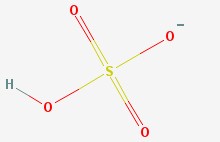
Hydrogen sulfate ion
There is the hydrogen sulfate ion (also called the bisulfate ion), which has a sulfur atom, four oxygen atoms, and a hydrogen atom. It has an extra electron (which makes it an ion) that hangs around one of the oxygen atoms.
If that lone extra electron attracts a proton (making a hydrogen atom) we get the other hydrogen sulfate. Because it has two hydrogens, it would be dihydrogen sulfate.
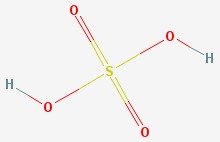
Sulfuric acid
A more common name for this molecule is sulfuric acid. This is a powerful acid that is widely used in industry. It is the acid used in lead-acid car batteries.
A similar sounding molecule is hydrogen sulfide.
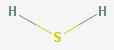
Hydrogen sulfide
Hydrogen sulfide is a poisonous gas, and is familiar as the smell of rotten eggs.
